Cell Viability Assessment
Label-free Cell Viability Assessment with DHM® Technology
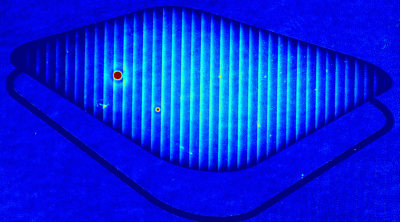
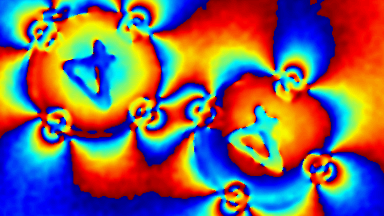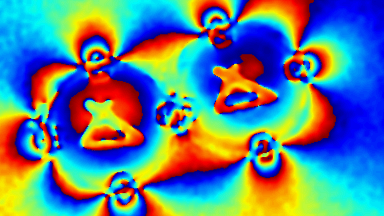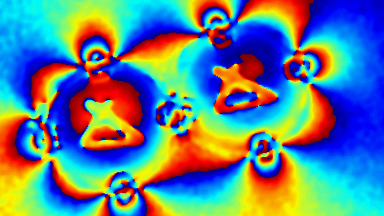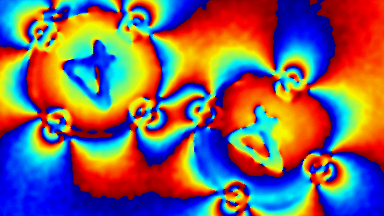
Lyncée Tec’s well-established label-free approach enables accurate and continuous monitoring of viable cell density based purely on morphological parameters, bypassing the need for invasive dyes.
Unique advantages of Lyncée Tec DHM® technology
- Continuous Monitoring: 190 fps recording provides instant feedback when culture conditions change, enabling timely interventions to safeguard culture health.
- Label-Free Advantage: Our technology avoids the use of external labels or dyes, preserving cell integrity and ensuring safety for therapeutic applications, including re-injection into humans.
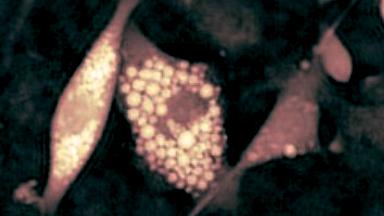
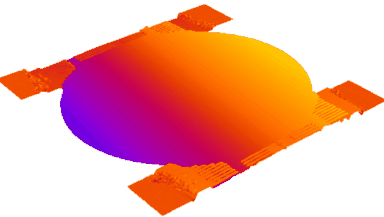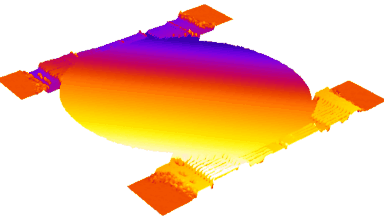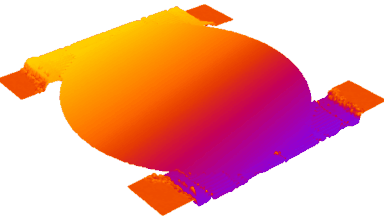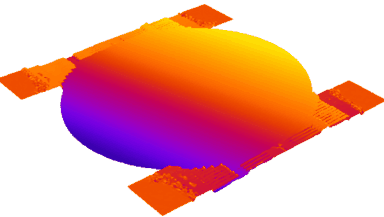
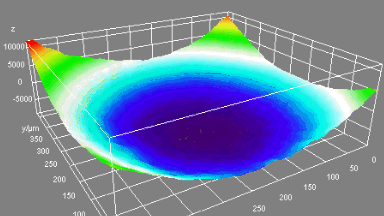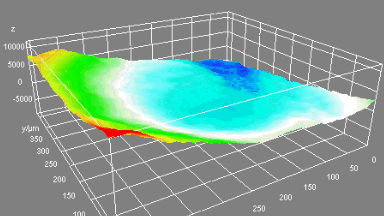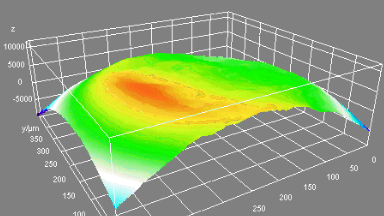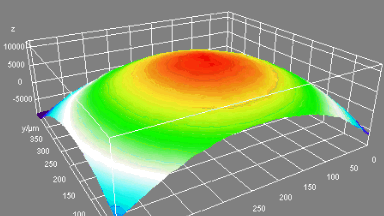
- Accurate VCD Measurement: Total and viable cell density are accurately calculated based on multiple morphological parameters directly measured on the recorded images.
- Additional CQAs: quantitative image analysis allows to extract additional Critical Quality Attributes (purity, phenotype, etc.) from the same dataset.
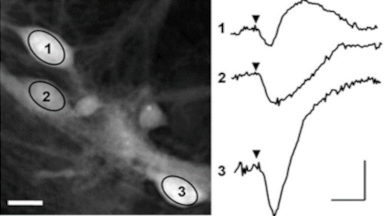
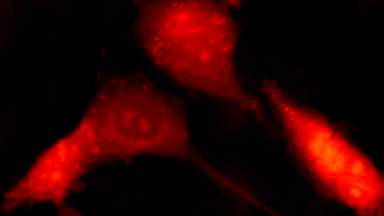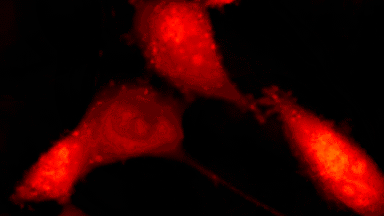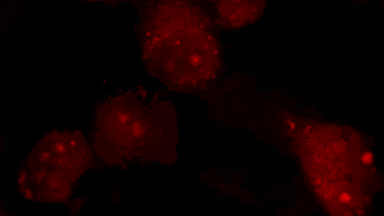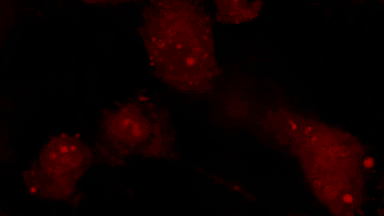
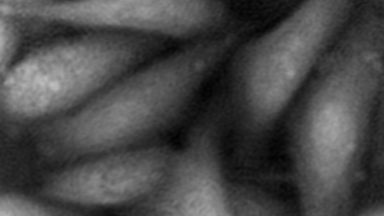
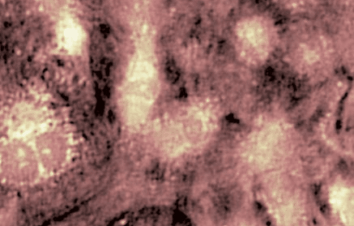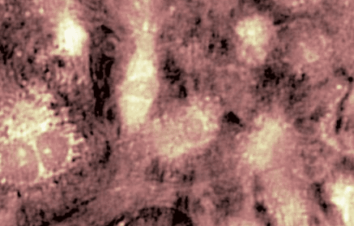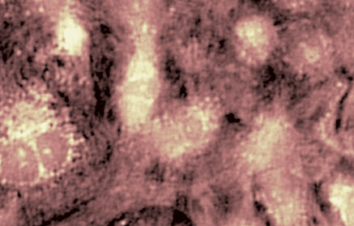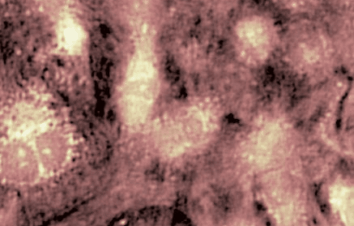
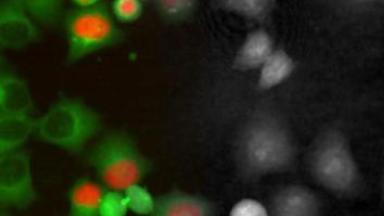
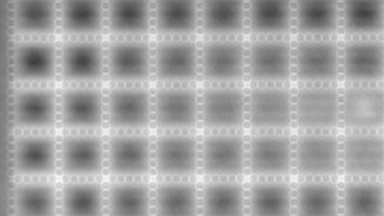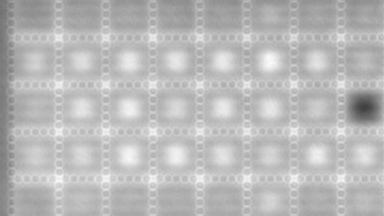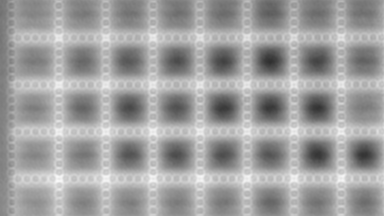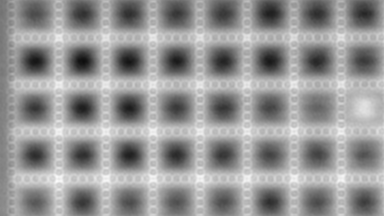
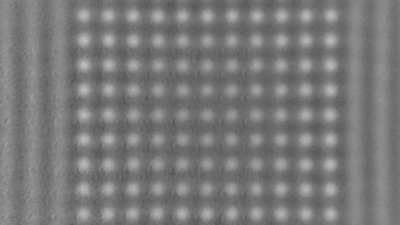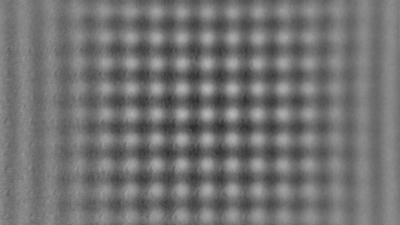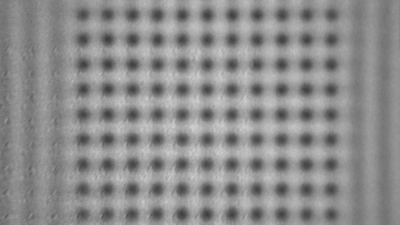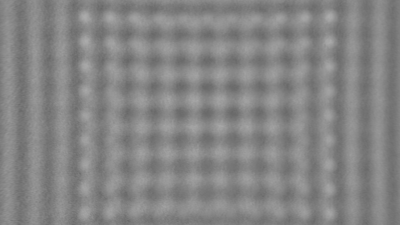
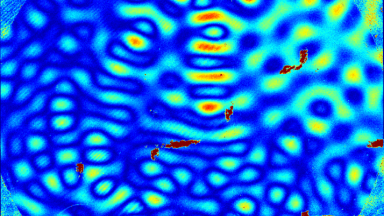
The superiority of DHM® VCD measurements has been validated through dual imaging techniques: quantitative phase imaging (QPI) and fluorescence microscopy. While fluorescence microscopy serves as the standard for identifying live and dead cells, a machine learning model developed by Lyncée Tec classifies cells based on their morphological traits from QPI images alone. This validation have shown that DHM® achieve an excellent correlation rate exceeding 84%, proving its precision and non-invasive capabilities.
This establishes DHM® as a essential tool for detailed, non-invasive cell analysis, potentially revolutionizing cell health monitoring in the field of cell and gene therapy.

Compatible DHM® products
- Standalone Transmission DHM: Suitable for standard applications.
- DHM-Camera Module: Enhances imaging capabilities in existing devices.
- OEM Custom Integration: Customizes DHM® technology to integrate seamlessly into your existing systems, enabling label-free cell viability measurement.