Ion Channels
Description
GABAA Receptor (Ligand-gated chloride Channel). Chloride channels represent a group of targets for major clinical indications. However, molecular screening for chloride channel modulators has proven to be difficult and time-consuming as approaches essentially rely on the use of fluorescent dyes or invasive patch-clamp techniques which do not lend themselves to the screening of large sets of compounds. In this application, GABAA mediated chloride currents have been monitored with DHM® and data are compared to electrophysiological records.
Material and methods
- Biological Model: HEK cells expressing α1, β2 and γ2s subunits of GABAA receptors (HEKGABA)
- DHM® solution: Non-invasive optical patch clamp of GABAA receptors
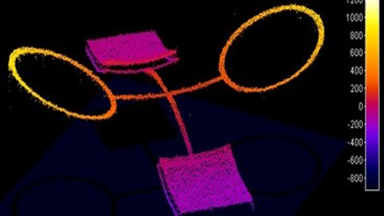
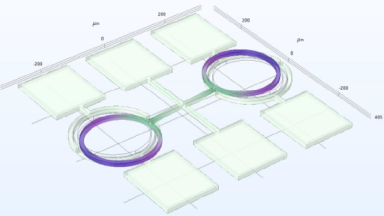
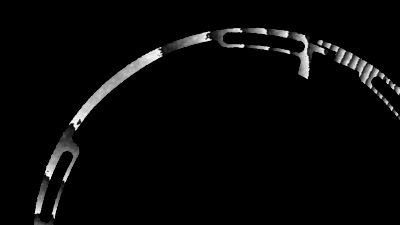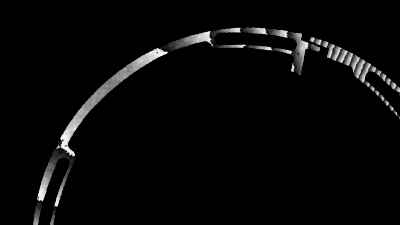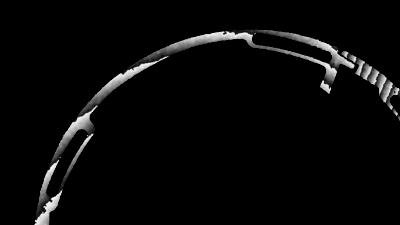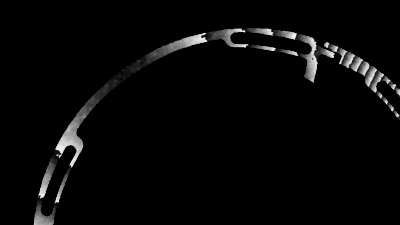
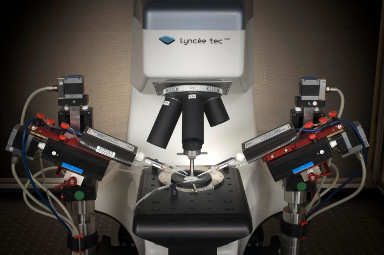
- Electrophysiology recording: Whole cell patch clamp system integrated around the DHM® (Illustration 1).
- Data analysis: Assessment of the optical patch clamp measurement by DHM® with recorded electrophysiological current.
Results
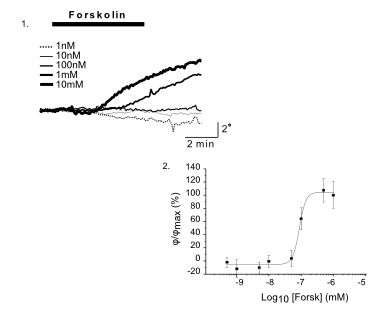
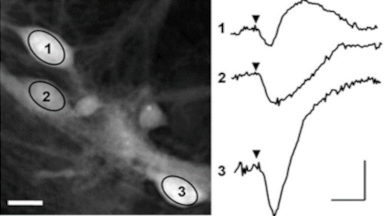
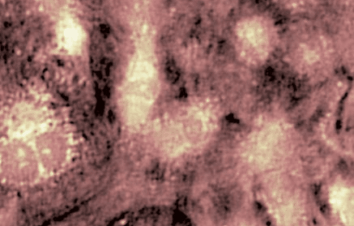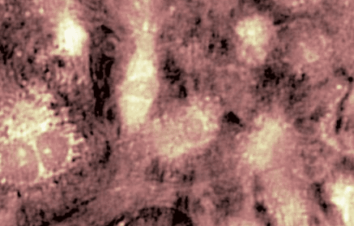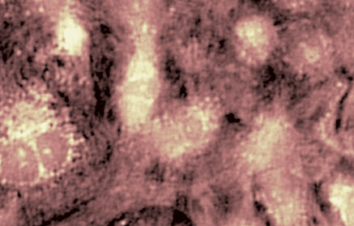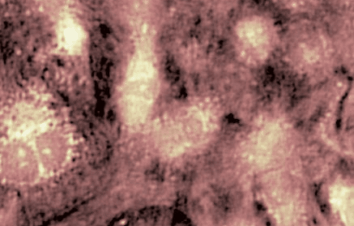
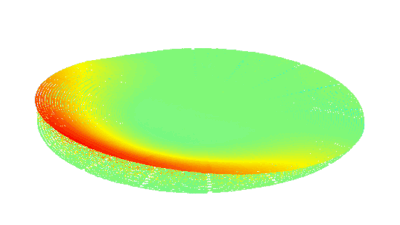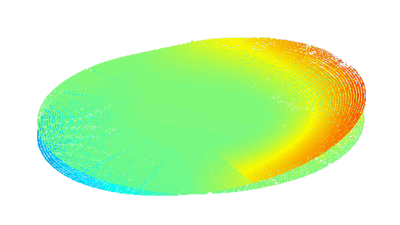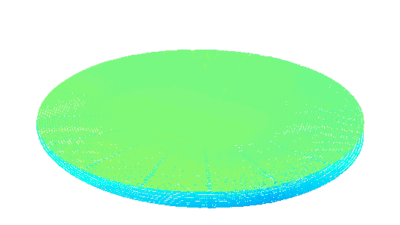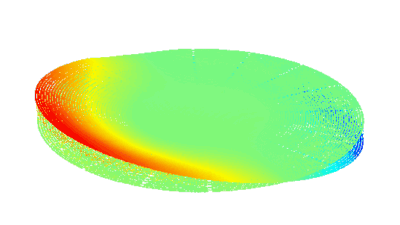
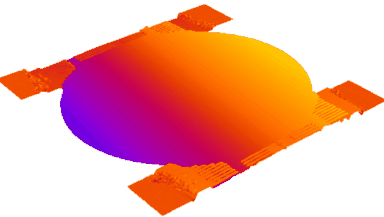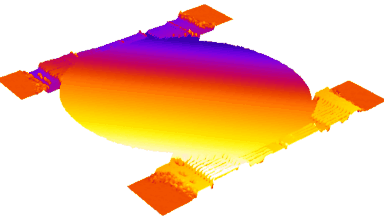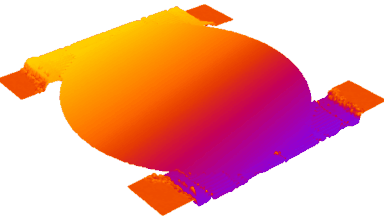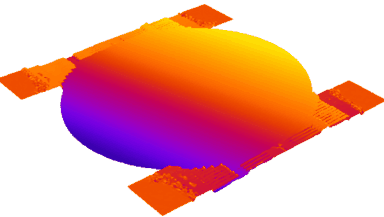
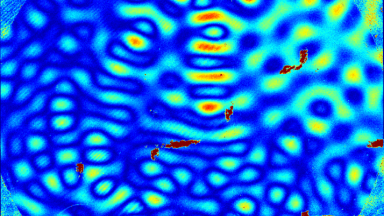
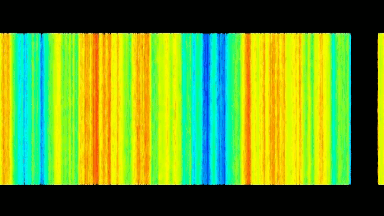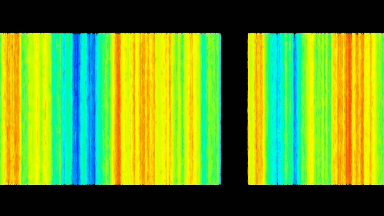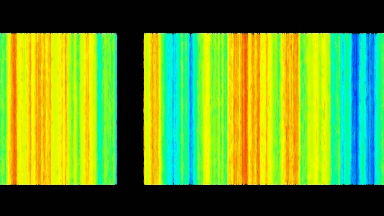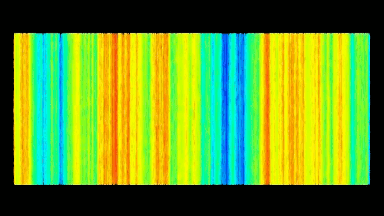
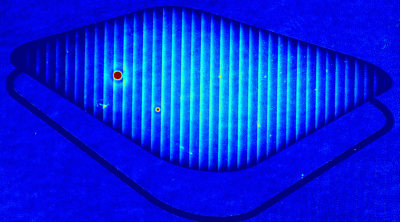
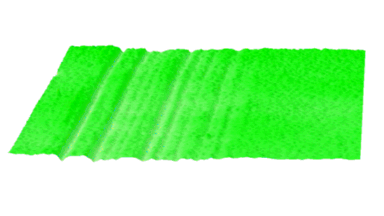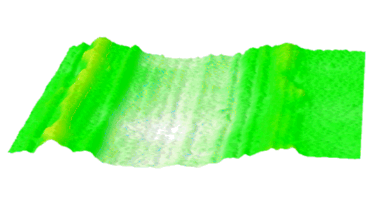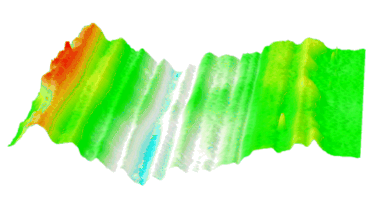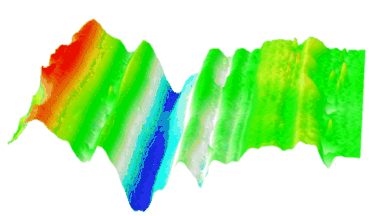
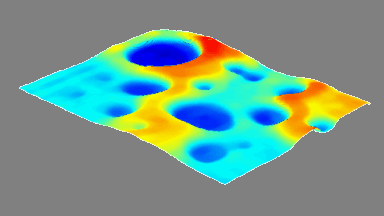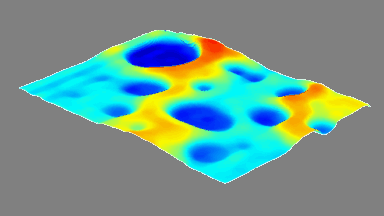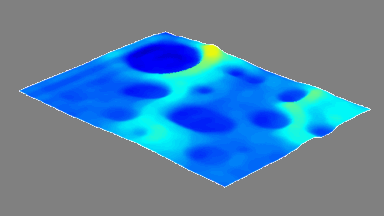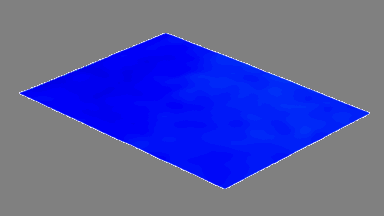
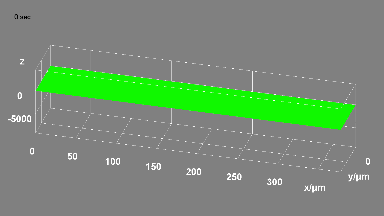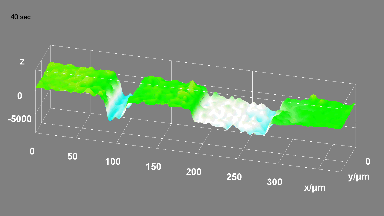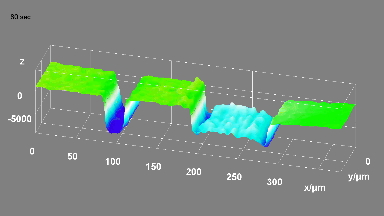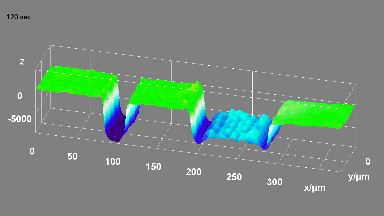
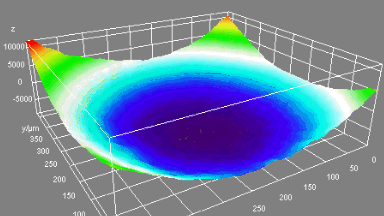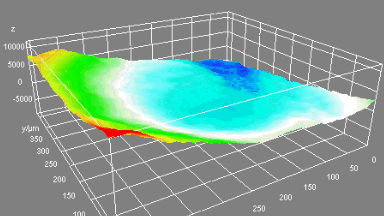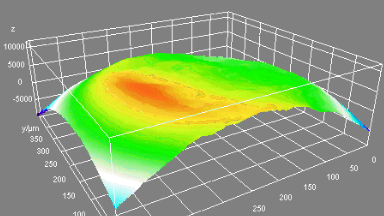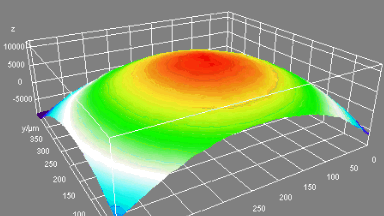
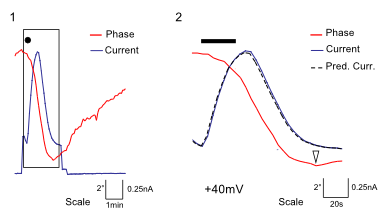
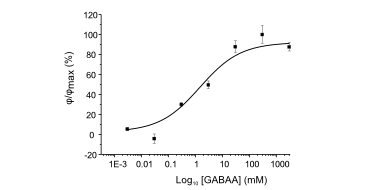
DHM® can non-invasively provide the quantitative determination of transmembrane chloride fluxes mediated by the activation of chloride conductance associated with GABAA receptors. Indeed through an original and patented algorithm, chloride currents elicited by application of appropriate agonists of the GABAA receptor can be derived from the quantitative phase signal recorded with DHM®. Moreover, pharmacological characteristics of GABAA receptors can be determined non-invasively and simultaneously on a large cellular sampling by DHM.
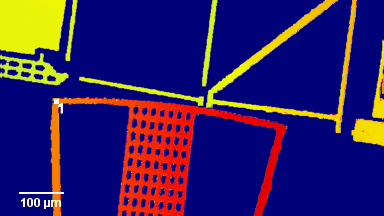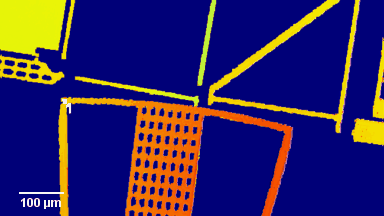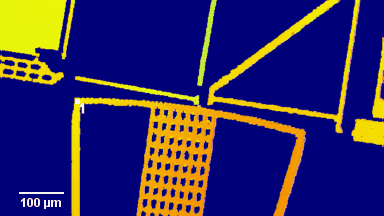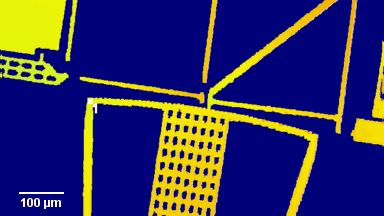
Figures 1 & 2 (adapted from Jourdain et al., PLoS One, 2012)
Publications
“I am proud to be one of the first owners of a DHM®.
The innovative data we generate with our microscope trigger major contributions to Optical electrophysiology.”
Dr Pascal Jourdain
Center for Psychiatric Neuroscience, CHUV university hospital, Cery,
Switzerland

Description
The ionotropic glutamate receptors (NMDA and non-NMDA) are involved in numerous physiological processes (neuronal development, memory, etc.) as in pathological processes (stroke, ischemia, neurodegeneration, etc.). The ionotropic glutamate receptors represent attractive therapeutic target in neurological pathologies.
Material and methods
- Biological Model: Primary cultures of cortical neurons prepared from E17 OF1 mice embryos of either sex
- DHM® solution: Non-invasive optical recording of NMDA and non-NMDA receptors
- Electrophysiology recording: Whole cell patch clamp system integrated around the DHM® (Illustration 1)
- Calcium Imaging: Calcium system integrated around the DHM® (Illustration 2)
Results
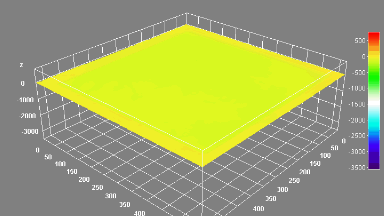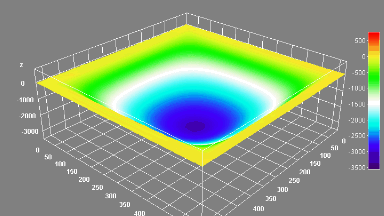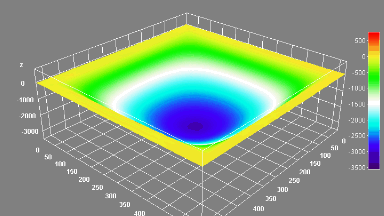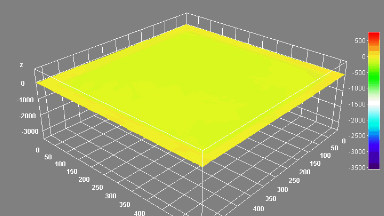
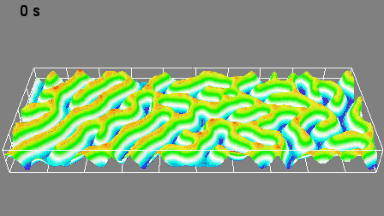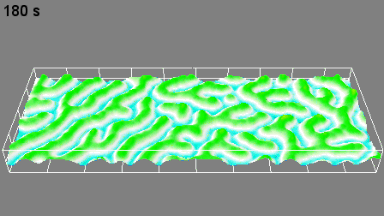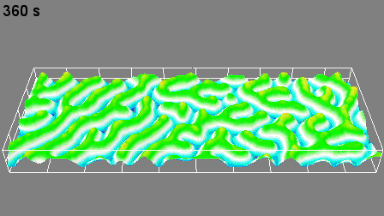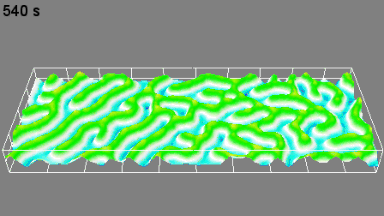
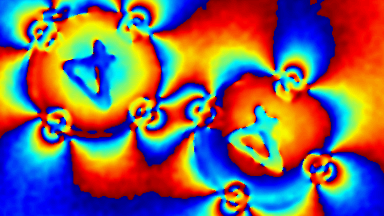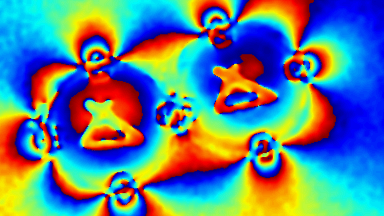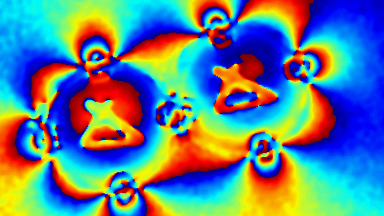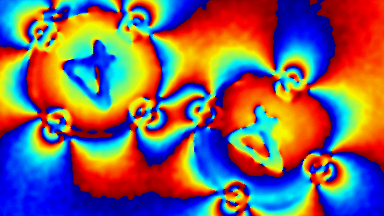
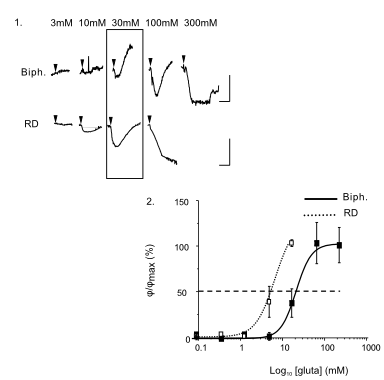
DHM® can detect in a dynamic and non-invasive manner the activity of NMDA and non-NMDA receptors providing an accurate indication of the physio-pathological status of neurons. Moreover, their pharmacological parameters can be determined very precisely for each type of ionotropic glutamate receptors.
Figure 3 (adapted from Jourdain et al., J. Neurosc., 2011)
Publications
Pavillon et al., JBO, 2010; Jourdain et al., J. Neurosc., 2011; Pavillon et al., PLoS One, 2012

Description
CFTR Protein (cAMP-activated chloride channel). The CFTR (Cystic Fibrosis Transmembrane Conductance Regulator) protein is an apical membrane protein functioning as a chloride channel regulating anion transport in secretory epithelial cells. A dysfunction of this protein in cystic fibrosis affects chloride, bicarbonate, sodium and water transports in epithelial tissue leading to thick and viscous secretions.
Material and methods
- Biological Model: CHO cells stably expressing the human form of CFTR protein (CHOcftr)
- DHM® solution: Non-invasive optical recording of CFTR protein activity
- Electrophysiology recording: Whole cell patch clamp system integrated around the DHM (Illustration 1)
- Iodide Efflux assays: The CFTR protein activity was assayed by measuring the rate of iodide (125I) efflux from CHOcftr
Results
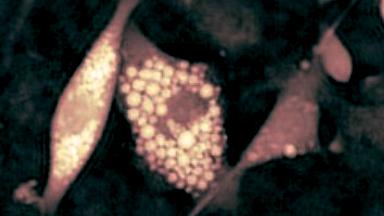
DHM® can detect non-invasively the activity of the CFTR protein and determine their pharmacological parameters within similar range than patch champ techniques or iodide efflux assays. Thus, optical patch clamp by DHM® provides a novel approach for the study of the functional properties of the target.
Figure 4 (adapted from Jourdain et al., JCS, 2014)
Publications