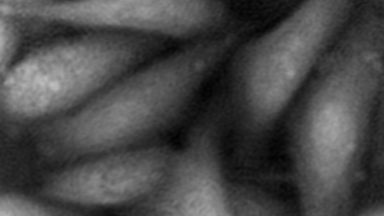
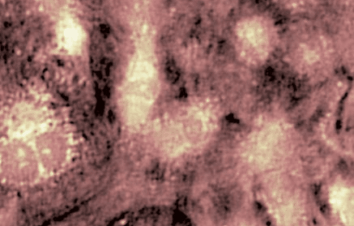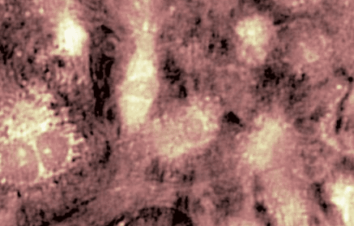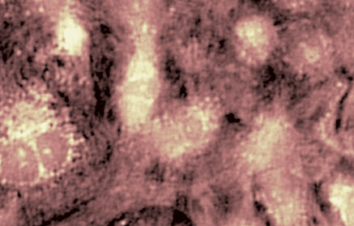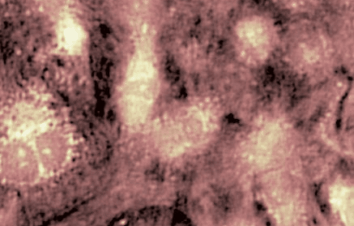
Transmembrane Water Flux
Transmembrane Water Flux solution by DHM® is the ONLY method for monitoring directly and in-situ trans-membrane water movements. The DHM® optical measurement provides quantitative measurement of water flux.
Strictly non invasive
- No use of fluorescent probes
- No use of radioactive tracers
- No phototoxicity
No need of sample preparation
- Measurement not affected by load and washout of labels
Well defined experimental protocol enabling high specificity
Measurements are performed in-situ, that is to say in native cells. There is no need to use any fluorescence, nanoparticle or radioactive tracers, or to add a complex and expensive set-up on top of your microscope. It applies to many experimental protocols and to many type of cells.
Cell volume regulation on astrocytes and neurones as well as on cystic fibrosis diseases mechanism involving CFTR protein and aquaporins has been demonstrated and published [Boss 2013, Jourdain 2014]
The transmembrane Water Flux solution combines a transmission DHM®, and a software for acquisition and analysis. It can be completed with an optional motorized XYZ stage and with a fluorescence module enabling you to compare DHM® measurements with fluorescence data. Output data are compatible with ImageJ for instance for more extensive and detailed analysis.
This novel possibility to measure quantitatively and non invasively intracellular protein concentration and transmembrane water fux opens new research possibilities. It enables to write more publications.
Innovative research possibilities
- Quantitative measurements of intracellular protein concentration
- Quantitative measurements of water movements
Efficient research
- No need of sample preparation and of contrast agents
- No delicate use of radiotracers
Transmembrane water flux monitoring solution based on DHM® is a simple method. It measures variations of concentration in real time, while other methods measure change of volume.
DHM® vs. Fluorescence
Fluorescence can be used for cell volume measurements, in conjunction with labeling. It provides an indirect evaluation of water flux activities. A drawback of this method is that the measurement accuracy can be affected by the loading protocol and photobleaching. Moreover, labels may affect the cells. With DHM®, the absence of labeling and the very low light intensity used prevent the measurement from these problems.
| Feature | DHM® | Fluorescence |
| Volume measurement | ||
| Concentration measurements | indirect | |
| No sample preparation |
||
| Ease of use |
DHM® vs. Total Internal Reflection Fluorescence microscope (TIRF)
TIRF enables to investigate the concentration in a thin region of a cells in contact with the substrate. Usually thicknesses thinner than 200 nm are sensed, using the evanescent wave . It is a local and indirect measurement, whereas water exchanges affect the whole cell.
| Feature | DHM® | TIRF |
| Volume measurement | ||
| Concentration measurements | indirect and local | |
| No sample preparation | ||
| Ease of use |
DHM® vs. Classical Interferometry
Classical Interferometry is difficult to perform on living cells due to coherence length and the thermal stability. It is a scanning technology (phase shifting). Measurements performed in reflection are difficult to interpret, and those performed in transmission have not been accessed through publications as DHM®. It is not a standard method.
| Feature | DHM® | Interferometry |
| Volume measurement | ||
| Concentration measurements | ||
| No sample preparation | ||
| Ease of use | phase shifting multiple measurements |
DHM® vs. Volume based methods
Volume based methods, in particular based on bio-impedance measurements, provide average measurements on multiple cells, and require usually dedicated cell plates. DHM® enables measurement on each individual cell on the measurement field of view on standard culture substrate.
| Feature | DHM® | Volume based methods |
| Volume measurement | averaged over cell population | |
| Concentration measurements | indirect | |
| No sample preparation | ||
| Ease of use | needs dedicated substrates |
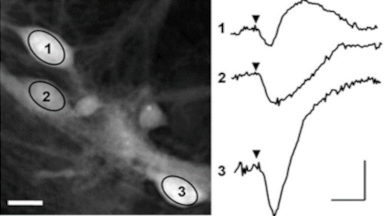
Transmembrane water fluxes through several channels of different type of cells have been investigated, and results have been assessed using pharmacology methods and patch clamp.
The passive or active transport of water through a plasma membrane is a concomitant of several cellular processes. Even if the functional involvement of water channels called aquaporin has been known for two decades, the trans-membrane water movements during cellular processes remain largely unknown. One of the reasons why this topic is still under-explored is the lack of reliable measurement techniques to directly quantify the trans-membrane water fluxes, at cellular scale, during a physiological (and pathological) situation. Application are shown on